
How to Profit – and Keep Profiting – From the GLP-1 Revolution
Dave Lashmet
Editor, Stansberry Venture Technology
One class of drugs is changing the world...
If you've been watching the news, you know what I'm talking about... safe and effective weight-loss drugs.
These drugs are already helping thousands of Americans. Soon, it'll be millions.
You see, Americans are growing and growing in the wrong direction...
In 1980, 12% of American adults – 1 in 8 people – were obese. Today, 42% of American adults – nearly half – are obese, and 9% of adults are morbidly obese. And it's not just the U.S. The rest of the developed world – including the U.K., Europe, and Japan – also has skyrocketing obesity rates.
Every year, the U.S. Centers for Disease Control and Prevention ("CDC") conducts a health survey of more than 1 million Americans to paint a picture of the nation's health. And the CDC's survey shows obesity is surging...
In 1980, the CDC says 15% of American adults were obese and 2% were extremely obese. By 2000, these rates had doubled with 30% obese adults and 4.5% extremely obese adults. And in 2020, 41.9% of adults were obese, with 9.2% of adults extremely obese.
The latest 2022 data shows that 22 states recorded adult obesity rates above 35%. That's up from 19 states in 2021.
And it's not just the U.S... A 2021 England health survey found 26% of adults were obese. Meanwhile, the European Union had a 16% adult-obesity rate in 2017.
In short, there are around 100 million obese adults in the U.S. and 15 million obese adults in the U.K., plus 60 million obese adults in Europe. With Canada, Australia, Japan, and South Korea, there are a total of 200 million obese adults in the developed world.
So obesity is a public health crisis. Being overweight taxes your heart, lungs, and mobility. It directly drives Type 2 diabetes, high blood pressure, liver failure, heart attacks, strokes, and osteoarthritis in your joints. Just as important, obesity limits your ability to do daily chores, manual labor, and exercise. This limits your strength, balance, and heart and lung health.
There is subtle damage as well, like severe snoring, which limits healthy sleep. This matters because it reduces your brain's self-cleaning – which adds to the risk of developing dementia.
But now, there's a solution... So in this report, we're covering Novo Nordisk (NYSE: NVO) and Eli Lilly (NYSE: LLY), two companies developing safe and effective weight-loss drugs. The Economist believes weight-loss drugs are a "trillion-dollar market." So we think these drugs could easily become blockbuster drugs.
As we'll explain, both Novo Nordisk and Eli Lilly are poised to address the colossal global market for obesity. And because the market is so vast, supply is the only real limit. So both companies can sell as much of the drug as they can possibly make for the next decade. That could lead to big gains for investors.
Let's get started...
The First Highly Effective Weight-Loss Drugs
We've been covering weight-loss drugs for years... It started with Novo Nordisk's obesity treatment – an injectable drug called semaglutide. Originally, semaglutide, under the brand name Ozempic, was developed to fight Type 2 diabetes – a disease that's driven by diet and obesity.
But Ozempic doesn't just cut blood sugar... It reaches the brain to boost your metabolism. It's also a novel appetite suppressant. Ozempic's Type 2 diabetes trial gave patients healthy weight loss without severe side effects. Plus, it homed in on fat – not muscle or vital organs. And there was a dose effect: The more you took, the faster you lost weight.
So Novo Nordisk tested a higher dose for prediabetic obese patients. This was a large-scale human clinical trial of high-dose Ozempic to treat obesity in 1,961 men and women.
At 20 weeks, the average patient on the drug lost 10% of their excess weight... At 62 weeks, 75% of patients lost 10% of their body weight and 35% of them lost 20% of their body weight. By the end, patients lost an average of 17% of their body weight.
There was also a "placebo effect" in the control group because these folks volunteered for the trial – they yearned to lose weight. But, on average, these patients only lost 3% of their body weight.
The U.S. Food and Drug Administration ("FDA") reviewed this data and approved the high-dose version of Ozempic, called Wegovy, for weight loss in June 2021.
For Eli Lilly, we've been following its tirzepatide drug. Tirzepatide was also developed for Type 2 diabetes, under the brand name Mounjaro. But once Eli Lilly saw that Mounjaro triggered weight loss, it tested the drug at a higher dose. Zepbound is the brand name of this drug for weight loss.
Eli Lilly's trial followed 2,500 obese patients for 72 weeks. That's 10 weeks longer than Novo Nordisk's trial. Since you steadily lose weight on tirzepatide, patients in this trial lost more weight than those in the Novo trial – with average losses of 15%, 19%, and 21% of body weight at three rising doses of tirzepatide.
Based on this data, the FDA approved Zepbound for sale in November.
In short, the trials show these drugs trigger profound and lasting weight loss, as long as you stay on drug. Nothing like these drugs' results has ever been seen before, except with surgery.
How to Design a $1 Trillion Drug
Wegovy and Zepbound work so well because they target the glucagon-like peptide-1 (GLP-1) receptor. GLP-1 is a hormone that occurs naturally in our bodies and helps regulate blood sugar. But Wegovy and Zepbound aren't the first drugs to target GLP-1. There are a lot of GLP-1 drugs.
Novo Nordisk's first-generation GLP-1 drug was called Victoza, and you had to inject it daily.
Eli Lilly's competing first-generation GLP-1 drug was called Trulicity. Its advantage was that you only had to inject it weekly. Among Type 2 diabetes patients, Trulicity was a blockbuster.
However, neither of these first-generation drugs triggered much weight loss.
Wegovy and Zepbound work better for weight loss because of how they fit into the GLP-1 receptor, the electrical charges they carry, and that they make it into your brain.
You see, the human body has thousands of receptors that listen for tens of thousands of unique signals. They also screen out false signals – including attacks from toxins or viruses.
Your receptors do this by having a highly particular three-dimensional shape for its binding pocket and requiring the right electrical bonds from whatever is in the pocket. It's like Goldilocks finding a chair that's "just right."
The image below shows the receptor these drugs are hitting at the billionth-of-an-inch scale. We made the receptors green and the two drugs gold and bronze
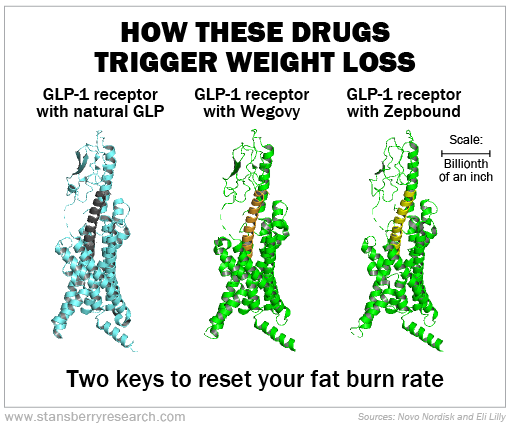
Now, designing a drug to fit a receptor's binding pocket doesn't mean the drug will work or that it won't cause side effects. That's why you run clinical trials. It's only by looking at a collection of patients that we can see the average expected results – plus how common or rare and how dangerous any side effects might be.
In this case, two side effects show us these weight-loss drugs are working. One side effect is a faster heart rate. Another side effect is loss of appetite.
You see, if it's cold outside or you're an extreme athlete, your body speeds up your base metabolism to burn energy. And if you also slow your appetite, you lose weight. Fundamentally, that's because fat storage prepares you for "famine," or any great energy expenditure. You store up energy in "feast" times – which in the modern world never ends.
All this doesn't play out in your stomach, though. These drugs don't simply make you feel fuller. Instead, they modify the basal energy-storage controls in your brain. In short, they reset your fat burn rate.
These are powerful signals. They can shape behavior, like rejecting a bowl of ice cream. That's how they're helping people lose weight.
One limitation is if you stop taking the drugs, then there's nothing holding you back from wanting ice cream. So your weight can rebound.
In short, these drugs can help solve the worldwide obesity epidemic.
Keeping Up With Demand
Given a 200-million-person market, the problem that Novo Nordisk and Eli Lilly face is supply, not demand.
The real trouble is, neither of these drugs are pills. They're protein drugs. Protein drugs have to be injected instead of swallowed. If you ate them, your stomach would break them up like it breaks up proteins in a hamburger. And you don't make protein drugs in a chemical factory. You need a living system.
Typically, this is a vat filled with yeast that's kept at a stable temperature. So it's similar to making beer. Kirin Brewery in Japan helped invent this biotech process. It's called a bioreactor system. Historically, the two biggest bioreactor-system projects for drugs were a $1 billion project by Amgen (AMGN) in 2016 and a $1.4 billion plant by Biogen (BIIB) in 2019.
To keep up with demand, Eli Lilly announced $10 billion in commitments to make more tirzepatide last year. Likewise, Novo Nordisk has a $6 billion factory plan for more semaglutide.
And in February, Novo Nordisk's parent company announced that it plans to spend another $16 billion to buy all the bioreactors at contract drug manufacturer Catalent (CTLT). Novo's cost will be $11 billion.
So Novo Nordisk plans to spend $17 billion on new factories to supply more Ozempic and Wegovy. And Eli Lilly is spending $10 billion to make as much Zepbound and Mounjaro as it can.
Not only is this 10 to 20 times more than any recent biotech project, it's basically as much as Budweiser's parent company spends on all its beer-making plants globally.
So you can see the scale that Novo Nordisk and Eli Lilly are working at. But the market doesn't get what this level of investment means for the future of these two companies. That's why we think Novo Nordisk and Eli Lilly have more upside potential ahead.
Before we dig into the numbers, Eli Lilly and Novo Nordisk also have "insurance plans"... So their upside isn't just dependent on these injectable drugs. Eli Lilly is developing an Alzheimer's protein drug that uses the same bioreactors as tirzepatide.
Meanwhile, Novo Nordisk is developing a second protein drug to cure obesity-driven fatty liver disease. That protein is called FGF21, but we nicknamed it "the salvation switch."
So neither Eli Lilly nor Novo Nordisk are "all in" on just these weight-loss drugs. Both companies have a fallback plan for these new protein-making plants.
Weight-Loss Drugs by the Numbers
The way we see it, the Economist's prediction of $1 trillion in revenue for the global weight-loss drug market must be spread over several years.
Still, based on known sales figures, we can see that these drugs are ramping up sales rapidly. For example, here's what happened in 2023:
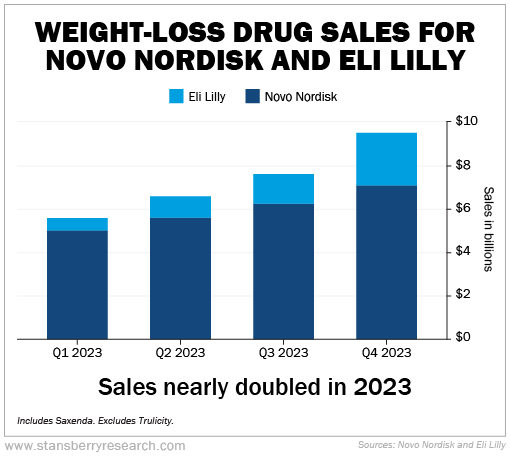
As you can see, weight-loss drug sales for Eli Lilly and Novo Nordisk hit more than $28 billion last year. More important, revenue nearly doubled across the four quarters.
Normally, we look at year-over-year figures. In the fourth quarter of 2022, Novo Nordisk and Eli Lilly sold $4.1 billion of weight-loss drugs – versus $9.5 billion in the fourth quarter of 2023. That's up 132%.
Being conservative for this year, if you project flat $9.5 billion quarterly revenue over 2024, that's $38 billion in sales for these two drugs this year. However, if sales double – like they did over the trailing 12 months – that would be $76 billion in sales this year.
Now, there will still be supply constraints in 2024. This is true even though Novo Nordisk is buying completed bioreactor plants for $11 billion. That's because you must run test samples of your production line to scale it.
So for 2024, it's more likely the weight-loss drug sales for these two firms will be more than $50 billion, or double what we saw in 2023. Once all the new plants come into production, we could see $75 billion in sales in 2025 and $100 billion in 2026.
Again, these are best-case scenarios. But we can reasonably predict that current production and sales growth rates will continue. At a flat $100 billion per year, it would still take 10 years to hit the $1 trillion the Economist envisioned.
And that raises the question of competition...
By one count, there are 42 rival drugs in development. But that doesn't mean they'll all work. For example, Pfizer (PFE) took two small-molecule drugs for weight loss – traditional pills – into mid-stage clinical trials. Both Pfizer drugs failed because each one had different, dangerous side effects.
The real threats are still at least five years out. And Eli Lilly has the most promising weight-loss pill in development. And Eli Lilly has no reason to get into a price war with Novo Nordisk. Right now, these drugs cost more than $10,000 per patient per year in the U.S. That's not likely to change any time soon
So over the next five years, we expect the Eli Lilly and Novo Nordisk duopoly in weight-loss drugs to continue.
But that's not all...
What Comes Next
Novo Nordisk and Eli Lilly are working to expand the approved indications (the medical conditions that a drug treats) for semaglutide and tirzepatide.
Remember, this has already happened twice. Ozempic went from a Type 2 diabetes drug to a weight-loss drug. And Mounjaro took the same path.
Novo Nordisk is testing semaglutide for Alzheimer's disease. You see, weight loss reduces blood pressure and spares blood vessels in your brain that could leak and cause senility.
As we said above, Novo is also running a dual trial with Wegovy and FGF21 to treat acute fatty liver disease.
Most important of all, Novo Nordisk recently completed a huge clinical trial in heart patients to see if weight loss reduced their rate of heart attacks, stroke, and death.
This was called the Select trial, and top-line results came out last August. The FDA announced on March 8 that Wegovy has won a label expansion to treat heart conditions, because the data is so great.
Specifically, this trial tested 17,600 overweight and obese heart patients. The drug reduced the risk of nonfatal heart attacks by 28% and death rates by 19% in 33 months of controlled testing.
Overall, there were 8,800 people per each side of the trial – on the drug versus a placebo pill. So there's a lot of statistical data to prove that Wegovy helps people.
More than likely, Eli Lilly's heart-risk outcomes trial for tirzepatide will show a similar benefit, since both drugs trigger weight loss using the same GLP-1 receptor target – just at different doses.
Any other GLP-1 drug would first have to win the right to treat obesity and also run trials for heart attack prevention. So Novo Nordisk has first-mover advantage... while Eli Lilly has second-mover advantage. It's unlikely even over the next 10 years that anyone else will catch up.
So Novo Nordisk and Eli Lilly have a 10-year advantage in a market that will soon be worth $100 billion per year.
And these products have huge margins – around 80% – despite the high cost of brewing proteins versus making drugs chemically.
Eighty percent margins on $1 trillion of sales could net Eli Lilly and Novo Nordisk $800 billion in net profits over the next decade. And both firms have third-generation drugs in development.
That's why we think that both Novo Nordisk and Eli Lilly can double and redouble in market capitalization over the next few years. And investors could see hundreds-of-percent gains.
Good investing,
Dave Lashmet
Editor, Stansberry Venture Technology
P.S. As I hope I made clear, the GLP-1 revolution is moving FAST.
Just like the early days of the tech boom... or with AI in the past few years... you either have the conviction to position yourself right now to be among the truly big winners – or you'll miss it.
So, for my specific buying instructions for NVO and LLY, along with SIX additional recommendations with up to 1,000% upside potential…
I hope you’ll consider claiming a 30-day trial to my work.
My publisher has arranged an exclusive offer for all Porter & Co. subscribers which you can find here.
About Dave Lashmet
Dave Lashmet is editor of Stansberry Venture Technology, an advisory service focused on the most important trend in the world - emerging technologies. This letter takes a "venture capitalist" approach to investing... seeking out stocks with strong catalysts and outstanding breakout growth potential.
Dave undertakes an intensive research process to discover under-the-radar technology, biotechnology, and medical companies with stunning catalysts for near-term growth. The stocks recommended in the Stansberry Venture portfolio have the potential to double and triple in price.
Dave was one of the first employees at Stansberry Research, back in the early days of the business. His unique insight into new technologies is responsible for some of the biggest gains in the history of the firm... including one of our top 10 "Hall of Fame" recommendations, Nvidia (1,466% gains).
Dave has spent 10+ years teaching and writing about medicine and technology at major research universities, and he's done follow-up research at some of the most important facilities in North America: Harvard Medical School, Johns Hopkins, MIT, and the Centers for Disease Control, just to name a few. He is also an inventor on three issued U.S. patents (in high-tech hardware and software), and a co-inventor of three more patent applications currently under review by the U.S. Patent and Trademark Office.
